Our NANOp2Lysis® platform
The NANOp2LysisTM platform allows the formulation of biological molecules to ensure a longer duration of action. The biological molecule is nano-precipitated and encapsulated in a polymer which allows high protection of the biological molecule during the transport toward the targeted tissue and a gradual release.
Therefore, the NANOp2Lysis technology unveils the full efficacy of the molecules allowing to reduce the number of injection and the dose used. The choice of the polymer is driven by the indication and target of the thrombolytic treatment.

A unique delivery technology preserving the 3D structure of biological molecule
A critical problem with thrombolytics is that most thrombolytic drugs are currently administered by frequent injections due to their short half-life in vivo. Extended-release technology offers the promise for reducing dosing frequency, maximizing the efficacy–dose relationship, and decreasing adverse side effects. However, developing extended-release dosage forms of proteins is not easy because most of manufacturing process cause proteins to denature.
The patented NANOp2Lysis® technology offers advantages thanks to its capacity to convert biological molecule to solid particles prior to encapsulating these biologicals molecules into polymeric systems.
This technology makes it possible to reduce the number of injections and the doses used
The NANOp2Lysis® platform allows the formulation of biological molecules to ensure a longer duration of action. The biological molecule is nano-precipitated and encapsulated in a polymer which allows high protection of the biological molecule during the transport toward the targeted tissue and a gradual release. Therefore, the NANOp2Lysis® technology unveils the full efficacy of the molecules allowing to reduce the number of injection and the dose used.
A very adaptative technology to feed a large pipeline of product
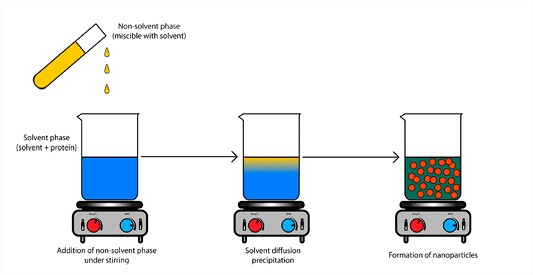
The nanoprecipitation technique of the NANOp2Lysis® platform is an easy to perform technique based on the non-solvent precipitation method that can be easily scaled up and which results in instantaneous formation of solid nanoparticles. The method requires the use of two solvents that are miscible with each other and results in spontaneous formation of nanoparticles on phase separation.
The solid nanoparticles are then dispersed in a polymer matrix to allow the controlled and extended release over the desired period.
OPTPA
A safer thrombolytic agent
Thrombolytic agents of the “plasminogen activator” family activate plasminogen, an inactive endogenous proenzyme, into plasmin, an active form that breaks down fibrin and dissolves the blood clot. For this reason, tPA has been developed as a medicine in the early 80’s to treat heart disease en stroke (90’s). Even today, thrombolytic therapy offers several advantages including cost effectiveness, use in any hospital (reduced “door-to-needle” time).
However, numerous side effects have also been described with tPA administration in the treatment of ischemic stroke, including hemorrhagic transformation. Experimentally, many groups have demonstrated an excitotoxic and inflammatory role for tPA in the treatment of stroke.
The patented Optimized tPA (OptPA) has been engineered on these observations. OptPA is a variant of tPA devoided of side effects described ahead, while conserving a high thrombolytic activity. OptPA is safer than tPA.
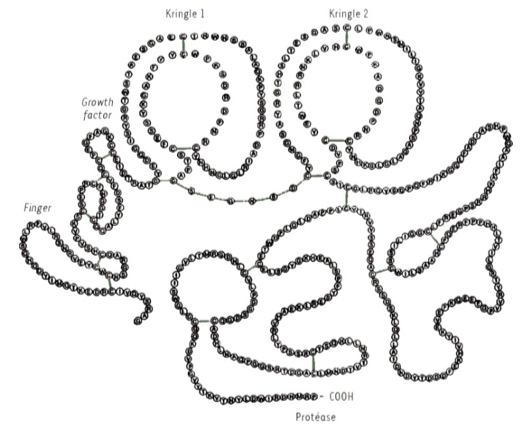
Tissue plasminogen activator primary sequence. OptPA is derived from tPA and bear two point-mutations, replacement of tryptophan by Arginine in position 253 and replacement of Arginine by Serine in position 275.
REFERENCES
Thiebaut, A. M., Louet, E. R., Ianszen, M., Guichard, M. J., Hanley, D. F., Gaudin, C., & Parcq, J. (2023). O2L-001, an innovative thrombolytic to evacuate intracerebral haematoma. Brain, 146(11), 4690–4701. https://doi.org/10.1093/brain/awad237.
Goulay, R., Naveau, M., Gaberel, T., Vivien, D., & Parcq, J. (2018). Optimized tPA: A non-neurotoxic fibrinolytic agent for the drainage of intracerebral hemorrhages. Journal of Cerebral Blood Flow & Metabolism, 38(7), 1180–1189.
Parcq, J., Bertrand, T., Baron, A. F., Hommet, Y., Anglès-Cano, E., & Vivien, D. (2013). Molecular requirements for safer generation of thrombolytics by bioengineering the tissue-type plasminogen activator A chain. Journal of Thrombosis and Haemostasis, 11(3), 539–546.
Giteau, A., Venier-Julienne, M.-C., Marchal, S., Courthaudon, J.-L., Sergent, M., Montero-Menei, C., Verdier, J.-M., & Benoit, J.-P. (2008). Reversible protein precipitation to ensure stability during encapsulation within PLGA microspheres. European Journal of Pharmaceutics and Biopharmaceutics: Official Journal of Arbeitsgemeinschaft Fur Pharmazeutische Verfahrenstechnik e.V, 70(1), 127–136.
Paillard-Giteau, A., Tran, V. T., Thomas, O., Garric, X., Coudane, J., Marchal, S., Chourpa, I., Benoît, J. P., Montero-Menei, C. N., & Venier-Julienne, M. C. (2010). Effect of various additives and polymers on lysozyme release from PLGA microspheres prepared by an s/o/w emulsion technique. European Journal of Pharmaceutics and Biopharmaceutics, 75(2), 128–136.