O2L-001
O2L-001 is the first product from the NANOp2Lysis® platform. O2L-001 is a patented therapeutic solution to treat patient suffering hemorrhagic stroke, an unmet medical need. The objective is to have a rapid and safe removal of the hematoma, that has formed in the brain following the bleeding event, leading to the reduction of the disability and mortality associated with the acute phase of hemorrhagic stroke.

MisTIE as a starting clinical proof of concept
To achieve this goal, the thrombolytic agent must be injected into the solid blood clot formed following the bleeding event to cause the degradation of the fibrin and to dissolve the blood clot. A critical problem with thrombolytics is that most they should be administered frequently injections due to their short in vivo life. This are the lessons learned from the MisTIE trial using recombinant tPA (alteplase) as the thrombolytic drug.
The choice of the polymer is driven by the indication and target of the thrombolytic treatment.
A disruptive in vivo gelation system to overcome standard thrombolytic limitations
Extended-release technology offers the promise for reducing dosing frequency, maximizing the efficacy–dose relationship, and decreasing adverse side effects. In O2L-001, our safe thrombolytic, OptPA, has been converted in solid nanoparticle thanks to our NANOp2Lysis® technology. The solid nanoparticles of OptPA are then dispersed in a polymer matrix to allow the controlled and extended release over a few hours of the OptPA inside the blood clot.
The extended-release technology selected for O2L-001 is an in vivo gelation system. The solid nanoparticles of OptPA are added to the fluid form of the polymer prior to administration. This OptPA-carrying polymeric fluid converts to gel form immediately after injection into the clot and encapsulates the OptPA in a depot. The mechanism for in vivo gelling is a reversed thermal gelling mechanism with temperature increase. The in vivo gelation system of O2L-001 is attractive due to its formulation simplicity and organic solvent-free condition.
The choice of the polymer is driven by the indication and target of the thrombolytic treatment.

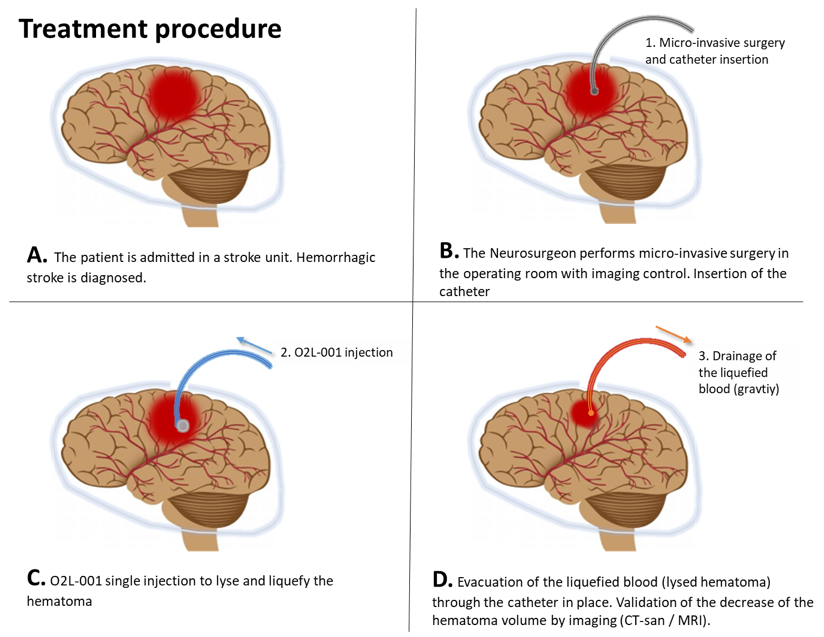
Easy to use, safe and effective
The fluid form of O2L-001 is injected into the core of the blood clot inside the brain by a minimum invasive surgery using a thin catheter. O2L-001 ensures then a rapid and safe removal of the hematoma.
- EASY, simplified model of administration (unique injection)
- EFFICACY, better thrombolytic effect on best translational model (vs gold standard)
- SAFE, reduced side effect (bleeding and neurotoxoxicity vs gold standard)